Abstract
Introduction: Vodobatinib, a selective novel BCR::ABL1 inhibitor effective against wild-type and mutated BCR::ABL1 isoforms was evaluated in Phase 1 open label study enrolling pts progressing or intolerant to ≥ 3 prior TKIs (<3 TKIs if TKIs not clinically feasible, advisable or approved; presence of T315I mutation exclusionary; NCT02629692). This sub-group analysis evaluates efficacy and safety of vodobatinib in CP-CML pts enrolled in the Phase 1 study, according to lines of prior TKIs received.
Methods: Pts were administered vodobatinib once daily in 28-day cycles at doses ranging from 12 - 240 mg, and included intra-patient dose escalations. Sub groups evaluated included pts receiving ≤ 2 prior TKIs (2T), ≥ 3 prior TKI (3T), ≥ 3 prior TKI including ponatinib (PON cohort, with or without asciminib) and ≥ 3 prior TKI including PON and asciminib (ASC cohort). Efficacy assessments included hematological, cytogenetic and molecular responses. All AEs were assessed as per CTCAE v4.03.
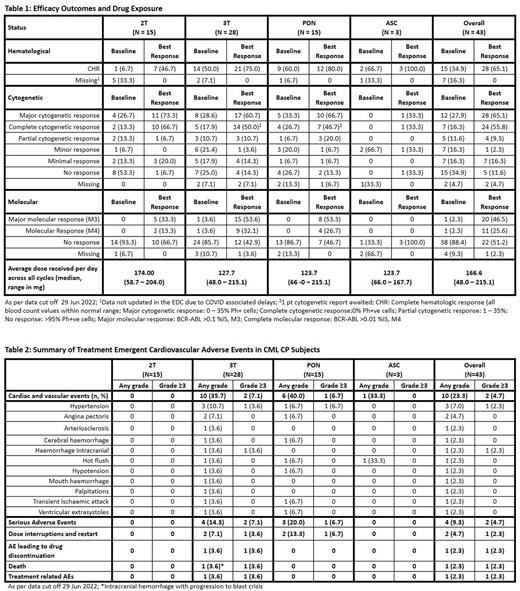
Results: At data cut off 29 Jun 2022, 43 pts were enrolled; 15, 28, 15, and 3 pts in 2T, 3T, PON, and ASC, respectively. Median age was similar for all sub-groups (61.5 - 67 y) except for 2T (48 y). Time since diagnosis was similar across subgroups (127.7 - 145.4 mo), except ASC (58.7 mo). Fifteen pts had baseline mutations. Nineteen (44.1%) and 24 (55.8%) pts were intolerant and resistant at baseline.
At baseline, 2 (13.3%), 5 (17.9%) and 4 (26.7%) pts in 2T, 3T, and PON respectively were in complete cytogenetic response (CCyR); all maintained it on vodobatinib, and 2, 4, and 4 pts in 2T, 3T, and PON, respectively, improved to MMR. On study, 8 (53.3%), 9 (32.1%), 3 (20.0%) and 1 (33.3%) pts in 2T, 3T, PON, and ASC respectively achieved CCyR on vodobatinib (Table 1). Major molecular responses (MMR) was achieved in 5 (33.3%), 14 (50.0%) and 8 (53.3%) in 2T, 3T and PON, respectively. Of the 20 pts with MMR as best response, 10 (23.6%) achieved molecular response M4 (BCR::ABL1 >0.01% IS). CCyR was achieved by 10 (23.3%) and 7 (16.3%) intolerant and resistant pts, respectively. Improvement to MMR was observed at doses ≥ 126 mg of vodobatinib in 13/32 pts. The median duration on study (overall median 31.2 mo, range 1-63 mo) and relative dose intensity (overall median 166.6 mg, range 48 - 215.1 mg) was similar for all groups. At data cut off, 2 pts have completed 5 years of treatment with sustained response. Of the 16 pts who experienced disease progression on study, 2 pts developed compound mutations (1 pt had F317L and H396R;1 pt had F317L H396R and M351T).
The most common treatment emergent adverse events (TEAEs) observed in ≥ 10% pts across sub groups included hematological toxicities (thrombocytopenia, neutropenia and anemia), gastro-intestinal toxicity (diarrhea, nausea, vomiting), and general disorders (myalgia). Most frequently observed Grade 3/ 4 AEs included thrombocytopenia (⁓ 14 % across 2T and 3T), neutropenia (observed in 2 pts each in 2T and 3T). Serum amylase increase was seen in 4 pts in the 3T subgroup. Serum lipase increase was seen in 1 pt in 2T and 3 pts in 3T. No pt discontinued due to AEs of amylase and lipase increase. Cardiovascular adverse events (CVAEs) were seen overall in 10 pts. No CVAEs were seen in pts in the 2T subgroup. The CVAEs on the study were usually grade 1/2 managed by dose interruption (Table 2). Grade 3 and higher cardiovascular adverse events (CVAEs) were reported in 2 pts in the 3T subgroup (1 transient worsening of hypertension in pt in PON cohort; 1 intracranial hemorrhage with blast phase in CNS). Dose interruptions were observed in 33.3% (2PT), 53.6% (3PT), 73.3% (PON), and 33.3% (ABL) while dose modifications were observed in 35.7% (3PT), 46.7% (PON), and 33.3% (ABL). No dose modifications were observed in the 2T subgroup.
Three (20%) 2T pts and 12 (42.9%) 3T pts reported serious adverse events (SAEs). Deaths reported included 2 each in 2T and 3T respectively (2T - sudden death considered unrelated to vodobatinib, and suspected COVID 19; 3T - intracranial hemorrhage with progression to blast phase and disease progression).
Conclusion: Vodobatinib demonstrated efficacy and safety in 2T and 3T pts (including pts treated with ponatinib and asciminib) and is well tolerated. Vodobatinib remains a potential option for these highly refractory pts. A phase 2 study (NCT02629692) of vodobatinib is currently ongoing in CML pts failing ≥ 3 TKI including ponatinib.
Disclosures
Cortes:Gilead: Consultancy; Takeda: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Bristol Myers Squibb: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Sun Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Kartos: Research Funding. Kim:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Otsuka: Consultancy, Honoraria, Research Funding, Speakers Bureau; Il-Yang: Consultancy, Honoraria, Research Funding, Speakers Bureau. Alvarado:Jazz Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; FibroGen: Research Funding; BerGenBio: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; Astex Pharmaceuticals: Research Funding. Nicolini:Pfizer: Membership on an entity's Board of Directors or advisory committees; Sun Pharma Ltd: Consultancy; Incyte biosciences: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis Services, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; KARTOS: Consultancy. Apperley:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Research Funding, Speakers Bureau. Charbonnier:Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Deininger:Fusion Pharma: Consultancy; Medscape: Consultancy; Dispersol: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; SPARC: Research Funding; Leukemia Lymphoma Society: Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of Study Management Committee, Research Funding; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Part of Study Management Committee, Research Funding. de Lavallade:Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria; Pfizer: Honoraria; Incyte: Honoraria, Research Funding. Lucchesi:Sanofi: Consultancy, Speakers Bureau; BMS: Speakers Bureau; SOBI: Speakers Bureau; Pfizer: Speakers Bureau; Incyte: Speakers Bureau; Morphosys: Consultancy; Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Grifols: Consultancy, Speakers Bureau. Mauro:Sun Pharma/SPARC: Research Funding; AbbVie, Bristol Myers Squibb, Novartis, Pfizer, Takeda: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding. Vandenberghe:Kite, a Gilead Company: Honoraria; Becton Dickinson: Honoraria; BMS/Celgene: Honoraria; Johnson & Johnson: Honoraria, Research Funding; Miltenyi Biotec: Honoraria; Novartis: Honoraria. Yao:Sun Pharma Industries, Inc.: Current Employment. Inamdar:Sun Pharma Advanced Research Company: Current Employment. Dillu:Sun Pharma Advanced Research Company: Ended employment in the past 24 months. Sreenivasan:Sun Pharma Advanced Research Company: Current Employment. Chimote:Sun Pharma Industries, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.